THE ONLY TREATMENT THAT HAS A DRY SATURATION FOR THE ENVIROMENTAL DISINFECTION (AIR AND SURFACES)
PATENTED UNIQUENESS ECONOMIC EFFECTIVENESS CONVENIENCE
MikroAir ™ is an “Industry 4.0” patented and certified Class 3 professional device for high-level environmental disinfection, which exploits the dry micronisation process (particle fractionation) to generate 0.5 to 1.5µm colloidal micelles, thanks to the combination of two substances: MkActive™ activator and PoliDisin ™ disinfectant.
These three combined elements (MikroAIR™ + MkActive™ and PoliDisin™) produce results in terms of reduction and abatement of health-care associated infections (HAIs) in highly frequented areas or areas where the risk of infection is particularly high, which no product on the national and international market can offer, for these main reasons:
- Being extremely light, micelles move in all directions (Brownian motion), even against gravity, thus ensuring total dry saturation of the environment (without which the treatment would not be so effective).

With other technologies, the saturation of a room depends on its humidity, with the consequent damage we all know, especially to electrical and electronic equipment.
- Micelles become more resistant, to avoid exploding when getting in contact with the surface, thus prolonging air suspension to improve the product microbial activity. Other devices currently available on the market aerosolize the particles which merge when touching each other, further increasing their weight and volatility, which prevents the environment from being saturated.
- Contact time reduced by the negative polarity of the colloidal membrane taking advantage over the alternating prevalence of pathogen positive polarity.
- Non-toxic, a safe treatment which does not require any access or laminar flow restriction, which would prevent the staff from working and using the treated areas, as is the case with hydrogen peroxide devices.
- Blockchain-based traceability of applications with disinfection certificates issued directly by the device and printed by means of dedicated terminals, with information about the bottle used, name of the hospital and ward, date and time of treatment, progressive number of the application.

- Deferred or remote activation of the device, which means that the device can be turned on and used remotely via data interconnection.
- Application time 5 to 6 times shorter compared to other similar devices (mc 60 in 15 minutes against 90 minutes).
MikroAir™ kit consists of:

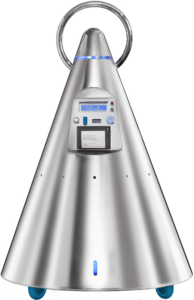
MikroAIR™: microniser, an innovative and very small (495mm X 750mm) medical device designed for air and surface disinfection through micronised applications. MikroAIR™ is equipped with a barcode reading system to recognise the barcodes printed on the bottles’ labels, as well as with other options to identify the room and the operator, so as to guarantee control and traceability of their use.
The device is also equipped with a printer that releases an application certificate at the end of each treatment, whose traceability results from a printout of the applications that will be delivered annually.

MK Active™: activator for the reconstitution of the powder disinfectant. This solution makes the aerosolized micelles more resistant, prolonging their suspension in the air and increasing their negative charge to facilitate pathogen attraction.

PoliDisin™: non-toxic powder disinfectant with potassium peroxymonosulfate and povidone-iodine; an innovative and convenient non-toxic and biodegradable product with virucidal, bactericidal, sporicidal, fungicidal properties.
I principali settori di applicazione sono:

Health care sector
Hospitals and polyclinics, Ambulances, Obstetrics clinics, Dental clinics, Scientific and clinical labs, etc.
PoliDisin™ AIR is easy to use, and, thanks to the instructions provided on the display, there is no room for error. The product can be easily applied in 4 steps:
- Reading the consumable’s barcode;
- Filling the tank with the consumable product (PoliDisin™ and MkActive™);
- Setting the timer or reading the room/operator code, and emission;
- Emptying to remove any residual solution.
Treatments can be set manually by entering the cycle duration based on the volumetry to be treated, or they can be programmed as deferred; in this case, the applications will start automatically.
The rooms can be accessed immediately after being treated.
Non-toxic treatment
The characteristics of the composition and the reactions that are developed in the solution ensure that the product is completely non-toxic to humans and the environment. There are no exposure limits and there is no need to wear special personal protective clothing.
Unique code for each individual bottle
Each individual bottle has a unique numeric code, ensuring complete traceability of the treatment.
Dry micronisation
Through micronisation (particle fractionation), colloidal micelles in the size of 0.5 to 1.0 µm are generated.
Ease of use
MikroAir ™ is characterized by extreme simplicity of use. Dedicated buttons for each function with an essential graphic interface
Total saturation of the treated room
The micelles are very light and move in all directions (Brownian motion) even against the force of gravity, ensuring a total dry saturation of the environment.
48V safe power supply
Decreasing the current has many advantages: conductors can be thinner and lighter; losses are decreased and efficiency increased. The switch to 48V emphasizes these advantages without bringing the voltage to a dangerous levels for users.
Block Chain type Certification
Steel device wheeled.
To effectively prevent the proliferation of bacteria, stainless steel surfaces are one of the most effective solutions as they counteract the formation the “biofilm”,an environment suitable for the growth of pathogens.
